Letter to the Editor: “Could bronchiectasis in post-acute sequelae of COVID-19 be reversible?”
by I Hutuca, L Sese, JF Bernaudin, PY Brillet, H Nunes, S Tran Ba (ioana.hutuca@gmail.com)
COVID-19 pneumonia imaging follow-up: when and how? A proposition from ESTI and ESRDear Editor in Chief,
With great interest, we read the propositions of the European Society of Thoracic Imaging (ESTI) and the European Society of Radiology (ESR) “COVID 19 pneumonia imaging follow up: when and how?” [1]. Indeed, we have been confronted on several occasions with the complexity of long-term COVID follow-ups especially regarding the use of the term “traction bronchiectasis” in COVID19 follow-up reporting. As mentioned in the article “the term traction bronchiectasis, suggesting irreversible fibrotic changes should be used with caution, as bronchial dilatation and distortion in areas of consolidation or ground-glass opacities may be reversible.”
In our recent experience with COVID19 follow-up, we noted the case of two patients that were initially reported as fibrotic changes that included traction bronchiectasis and who secondarily improved with partial reversibility of bronchial dilation on a latter control CT scan.
Our first patient was a 61-year-old man who developed a COVID 19 pneumonia after close contact with a SARS-COV-2 positive family member. After 14 days, the patient presented to the on-call room with acute exacerbation of dyspnea. The CT scan showed ground-glass opacities with an extent of pulmonary damage of 25-50%, and segmentary pulmonary embolism. As his condition continued to aggravate, he was transferred to the Intensive Care Unit (ICU) for acute respiratory distress syndrome (ARDS). He spent 3 weeks in the ICU where he was on mechanical ventilation and received corticosteroids.
An HRCT was performed 7 weeks after the disease onset, showing changes reported as traction bronchiectasis, reticulations, and ground-glass opacities. For this presentation, the diagnosis of post-COVID pulmonary fibrosis was suspected and corticosteroids were initiated as a therapeutic test. The patient had two HRCT scans to reassess the pulmonary lesions at 6 and 8 months. The first one revealed a decrease in ground-glass opacities, with the persistence of reticulations and bronchiectasis while the second one showed partial regression of bronchiectasis, especially in the lower pulmonary lobes (Fig 1).
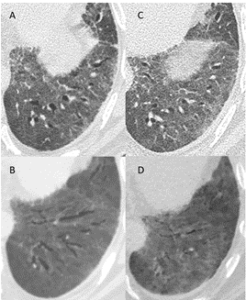
Figure 1: A, B: axial slices from a CT scan (A: 1 mm slice, B: minimal intensity projection reconstruction) obtained 6 months after the onset of symptoms, and 3 months after discharge from the ICU, showing traction bronchiectasis in the left lower lobe and the lingula. C, D: axial slices from a CT scan (C: 1 mm slice, D: minimal intensity projection reconstruction) obtained 8 months after the onset of symptoms, and 5 months after discharge from the ICU, showing reversion of the traction bronchiectasis
The second patient was a 59-year-old woman who presented to the emergency department with fever, nausea, and asthenia. An RT PCR test for SARS-COV-2 came out positive and had an HRCT which showed a moderate extent of ground-glass opacities (10-25%). As oxygen needs increased, the patient was transferred to the ICU where she received nasal high flow oxygen curative anticoagulation and corticosteroids for an ARDS.
An HRCT was performed at 3,5 weeks from disease onset, which showed bilateral pulmonary embolism, decrease in ground-glass opacities, and “fibrotic-like” lesions in the upper lobes with traction bronchiectasis. The patient was hospitalized in the ICU for a total of 6 weeks. At 4 and a half months of disease onset, the patient was seen at the outpatient clinic. The control HRCT revealed partial regression of fibrotic lesions and normalization of the bronchi caliber, especially in the upper pulmonary lobes (Fig 2).
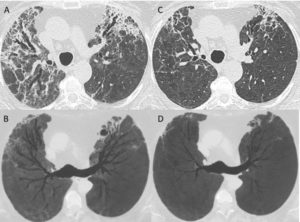
Figure 2: A, B: axial slices from a CT scan (A: 1 mm slice, B: minimal intensity projection reconstruction) obtained 6 weeks after the onset of symptoms, showing traction bronchiectasis in the upper lobes along with ground-glass opacities and fibrotic reticulations. C, D: axial slices from a CT scan (C: 1 mm slice, D: minimal intensity projection reconstruction) obtained 4,5 months after the onset of symptoms, showing partial reversion of the fibrotic changes and normalization of the bronchi caliber.
We do share the belief that the terms “traction bronchiectasis” and “pulmonary fibrosis” should be avoided in the first control CT scans before confirmation with long-term follow-up, favoring instead the more cautious term of “bronchial distortion”. Here is what we observed from the experience with the above cases and literature search.
Firstly, traction bronchiectasis are considered as a fixed remodeling of the lung parenchyma observed in various fibrosing interstitial lung disease. However, several situations cast doubt on the irreversibility of traction bronchiectasis. The majority of cases of reversible bronchial dilation found in the literature are in the pediatric population. In adults, reversibility of fibrosis and bronchiectasis has been seen in some cases of connective tissue associated lung fibrosis (especially in case of acute or subacute symptoms, according to our own experience) [2], non-specific interstitial pneumonia, chronic hypersensitive pneumonia, drug toxicity [3-5]. Most importantly for our cases, the fibroproliferative process post-ARDS has also shown to be reversible in some situations. As traction bronchiectasis are known to be a manifestation of pulmonary fibroproliferation in ARDS caused by SARS-CoV2, they undergo a remodeling process over time of the immature fibrosis occurring after diffuse alveolar damage [6] and is potentially responsive to steroid treatment [7].
Secondly, the most common radiological pattern in Post-Acute Sequelae of COVID-19 (PASC) is organizing pneumonia [8], which may be resolving in up to one year [1]. Additionally, drawing a parallel with the treatment of organizing pneumonia of other causes, corticosteroids have been promptly used in the treatment of ARDS caused by SARS-CoV-2 [9], as well as in the management of PASC. Initially, many clinicians have used corticosteroids in PASC empirically [10], but recent studies have shown favorable outcomes with an improvement of CT features (resolution of the more solid components) and pulmonary function after corticosteroid treatment [8]. Our patients had corticosteroid therapy both in the ARDS phase and in PASC management, this could be one of the factors that explain the partial resolution of bronchial dilation.
Lastly, from a pathological point of view, alveolar collapse is an important process involved in traction bronchiectasis in idiopathic pulmonary fibrosis. The main mechanisms involved are loss of epithelium and denudation of alveolar basal lamina that will be incorporated in the interstitial alveolar septum [11]. This process, called ‘collapse induration’, will lead to the inability of repopulating the basal lamina with regenerating epithelium. We can imagine that before incorporating the denudated basal lamina into the alveolar septum, some alveolar spaces can still be unfolded, leading to partial normalization of bronchial caliber.
We hope that these points will add up to the understanding of the potential reversibility of bronchial dilation. As mentioned in the article, reporting of changes in post-COVID 19 should be done with precaution, the term “bronchial distortion” being preferred to “traction bronchiectasis”.
