Reply to: Are all questions about the safety of MRI contrast agent answered?
by Marie-Luise Kromrey and Jens-Peter Kühn (marie-luise.kromrey@uni-greifswald.de)
Kromrey ML, Liedtke KR, Ittermann T et al. (2017) Intravenous injection of gadobutrol in an epidemiological study group did not lead to a difference in relative signal intensities of certain brain structures after 5 years. Eur Radiol 27: 772-777.Dear Editor,
We thank Dr. Goischke for his interest in our article about neuronal gadolinium deposition after intravenous application of macrocyclic gadolinium-based contrast agents detected by magnetic resonance imaging in a 5-year longitudinal survey (1, 2) and his critical comments.
The author of the letter to the editor asks “Are all questions about the safety of MRI contrast agents answered?”. In general, we cannot completely answer this question. However our data support previous investigations dealing with safety of the macrocyclic gadolinium-based contrast agent gadobutrol (3-5). The applied dose of gadobutrol (1.5 fold as normally used) did not lead to measurable changes in signal intensity in neuronal tissue 5 years after a one-time injection. Therefore, we assume gadobutrol in the used dosage to be safe.
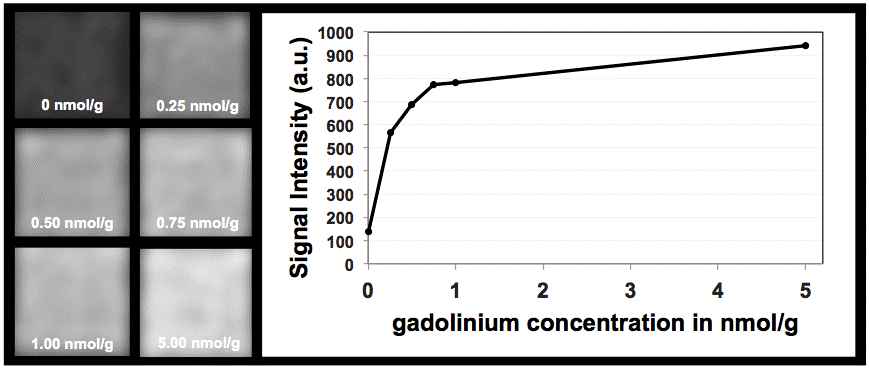
Dr. Goischke mentioned an important point – the sensitivity of MRI depending on field strength and the sequence technique used for data acquisition. For publication, we refrained from presenting all basic experimental details in the Materials and Methods section. Additionally, a phantom experiment was performed, in which a gadolinium phantom was generated including the following concentrations: 0 nmol/g, 0,25 nmol/g, 0,5 nmol/g, 0,75 nmol/g, 1 nmol/g and 5 nmol/g. The concentrations of the phantom were chosen in the knowledge of previous publications on neuronal gadolinium depositions in animals, using the linear chelate gadodiamide (6-8). In summery, we were able to demonstrate that the MRI (1.5T) and the used sequence technique (3D T1-weighted Magnetization Prepared Rapid Gradient Echo Sequence (MPRAGE, Siemens Healthcare)) are able to visualize signal intensity changes of very low gadolinium concentrations (see Figure below).
Furthermore, the author of the letter criticized that we did not investigate the clinical relevance and clinical manifestation, respectively, of neuronal gadolinium depositions. However, the clinical relevance of neuronal gadolinium deposition was not the purpose of our study. Our data were retrospectively observed in a healthy population and exclusively performed under scientific conditions.
Finally, previous investigations and our data support the assumption that the macrocylic agent gadobutrol does not lead to measurable gadolinium depositions in contrast to linear compounds.
If you would like to comment on this letter, please send your comments together with full contact data to office@european-radiology.org. Replies and comments will be reviewed by the Editorial Office and published on this website.